Supercritical water oxidation (SCWO) of sludge

Introduction to SCWO
Supercritical water oxidation refers to the oxidation of organics in water in the so-called supercritical phase of water. These conditions represent the most aggressive, in terms of temperature and pressure, of all thermochemical oxidation processes.
‘Supercritical’ refers to the state of water within a specific region of pressure (>218 bar) and temperature (>374 °C). The physical properties of water, specifically its density, viscosity, diffusivity, dielectric constant and its subsequent solubilisation of organic and inorganic compounds, change significantly in the supercritical region. As such, supercritical water is effective both as a reactant and a solvent.
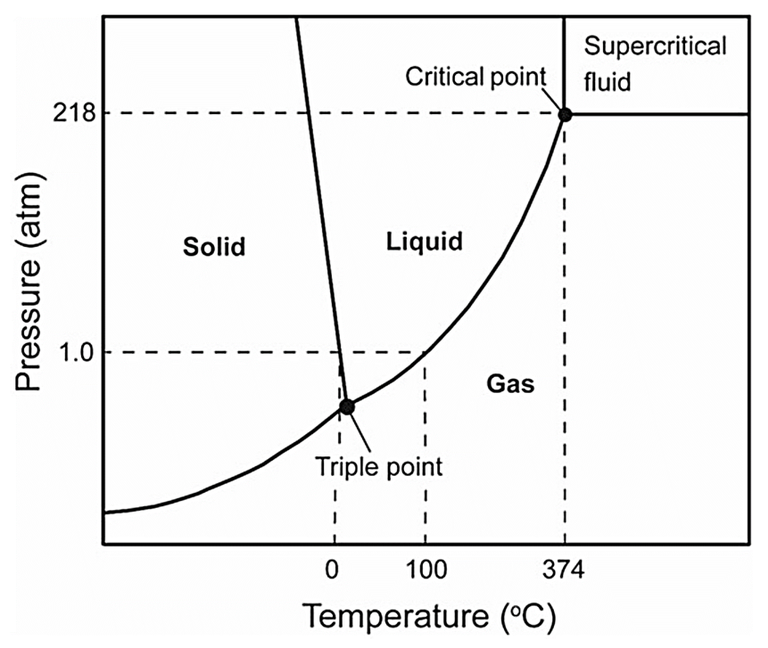
Supercritical conditions can be used for both oxidation (SCWO) and gasification (SCWG). In the oxidation mode, as with all oxidation reactions, degradation of the organic matter is enhanced by using an oxygen-enriched gas source as well as elevated temperatures and pressures. The process becomes autothermal, i.e. sustained by the oxidation process without the requirement for further heating, at around 10% sludge solids concentration (or 50 g/L COD) for typical municipal wastewater sludge.
SCWO was originally commercialised in the 1980s, but was subject to a number of operational challenges:
- corrosion, under the acidic conditions created,
- salt precipitation/accumulation from desolublised inorganic matter, and
- erosion from solid particles.
newsletterSignup is not templated
These challenges, and corrosion in particular, led to the decline in implementation of the technology. Most of the SWCO plants installed in the 1990s and early 2000s, primarily for treating challenging industrial effluents, have been shut down.
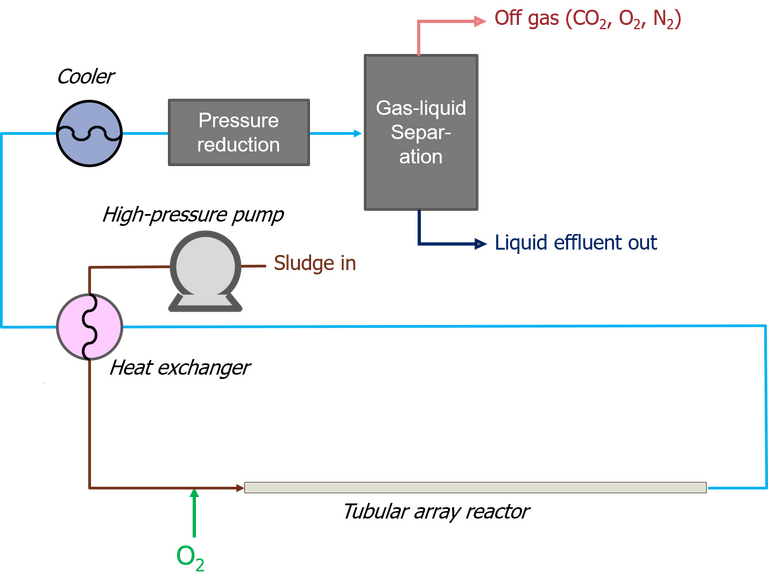
Of the few commercial SCWO technologies which currently exist, the Superwater and AquaCritox process technologies have both been successfully demonstrated against a municipal wastewater sludge feed. Both employ a pure oxygen gas source and a tubular reactor configuration.
The sludge is pressurised to ~260 bar, then heated to 250−300 °C. The subsequent oxidation reaction, which takes place in an array of steel tubes, may then raise the temperature to up to 600 °C, sufficient to mineralise the organic carbon within one minute. Organic nitrogen and sulphur are converted to nitrogen gas and sulphate respectively; the reactor temperature is apparently low enough to avoid NOx formation.