Sludge-to-energy recovery methods − a review

Jumoke Oladejo1, Kaiqi Shi1, Xiang Luo1, Gang Yang1
and Tao Wu1,2
1New Materials Institute, 2Department of Chemical and Environmental Engineering, University of Nottingham, Ningbo 315100, China.
Contact: jummyoladejo@gmail.com | Xiang.luo@nottingham.edu.cn | Gang.YANG@nottingham.edu.cn
1. Introduction
The need for safe and sustainable disposal routes for sludge becomes more acute with each passing year as the amount of global sewage sludge increases. Annual sewage sludge production has recently been estimated at 10 and 20 million tons dry solids (DS) in Europe and China respectively, with around 85% of the sludge generated in China being improperly discharged to water bodies.
Reuse of the stabilised sludge (i.e. sludge whose pathogen content and putrescence has been substantially reduced) for agriculture represents the most widespread reuse application. However, this route is constrained by increasingly stringent legislation governing the sludge quality for agricultural use – particularly with reference to trace amounts of toxic metals.
The volatile organic content of dried sewage sludge, which generally ranges from 21–48%, equates to a latent energy of 11–22 MJ/kg, which implies a calorific content comparable to and/or higher than lignite or various biomass materials. Energy recovery from sludge therefore represents an attractive, and indeed established, processing route for eliminating its volatile organic matter while recovering both energy and other resources. The latter include the end solid product and constituents such as nutrients, and phosphate in particular.
The selection and/or effectiveness of the energy recovery process is influenced by the sludge physical and chemical properties, and in particular the moisture, nitrogen and heavy metal content. Whilst anaerobic digestion (AD) and incineration are both established processes for recovering part of the sludge calorific content, more recently developed thermochemical technologies can potentially offer improved energy capture efficiencies and reduced environmental impact.
2. Technology comparison
2.1 Overview
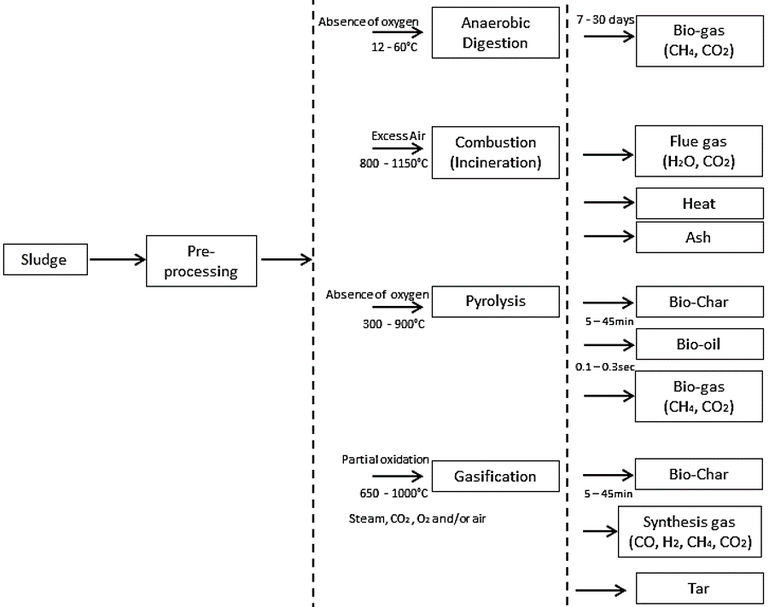
It is instructive to review some of the newer thermochemical technologies, specifically pyrolysis and gasification, and compare their process characteristics to those of the two established processes (Fig. 1). Several of these attributes can be highlighted:
- The latent energy of the different processes is recovered in different ways. For incineration, it is captured as heat through thermal oxidation (i.e. combustion). The heat can then be either used directly or converted to electrical energy using combined heat and power (CHP) engines, whereas the other three processes produce a liquid and/or gaseous biofuel.
- AD is a biological process, whereas the others are thermochemical. The AD process is therefore much slower and so the plants much larger in terms of land area.
- AD provides a relatively low conversion of the organic carbon, with 40–70% of the organics remaining in the solid residue (digestate) product fraction following treatment.
- The three thermochemical processes provide >80% elimination of the organic carbon and are considerably more rapid, with treatment times of minutes or even seconds compared to the 7–35d residence time of AD.
- AD generates a methane-rich (45–85%) biogas. Pyrolysis and gasification can respectively recover the latent energy predominantly as a pyrolytic oil or 'bio-oil' (from condensable, combustible gases) and hydrogen gas (the dominant constituent of the 'syngas' product stream from gasification).
- Pyrolysis and gasification are respectively conducted under oxygen-free or oxygen-depleted conditions and generate product streams with reuse potential (Table 1). The biochar product from these processes can potentially be used in higher-value applications than those appropriate to the solid ash residue from incineration.
- The three thermochemical processes require sludge pretreatment by dewatering and thermal drying to reduce the moisture content, whereas AD requires only sludge thickening as pretreatment.
| Source | Application |
|---|---|
| Tar/bio-oil | Reforming to syngas |
| Syngas/bio-oil | Conversion to liquid transport fuels or chemicals |
| Biochar | Gasification to syngas; combustion for thermal energy recovery |
| Biogas/syngas | Combustion for thermal energy recovery; upgrade to methane/hydrogen |
| Thermal energy | Conversion to electrical energy using turbines or CHP engines |
The dry solid (DS) fraction of the sludge is made up of:
- non-toxic organic compounds derived mostly from plant sources (characterised by a high volatile solids content), accounting for up to 48% of the DS and ~60% of the energy content in the raw wastewater and with a heating value of 11–22 MJ/kg
- micro-organisms, including pathogens
- non-toxic inorganic compounds of aluminium, silicon, iron, calcium-containing and other benign elements
- toxic inorganic compounds containing metals such as zinc, nickel, mercury and chromium and non-metals such as arsenic: these compounds are higher in concentration in sludge than in other solid fuels and derive mainly from industrial waste and corroded sewers
- toxic organic pollutants like dioxins and polycyclic aromatic hydrocarbons, and
- phosphorus- and nitrogen-containing compounds from peptides, proteins, sugars and fatty acids.
These constituents change physically and chemically with each energy recovery method. Such variations include reduction in organic content, fluctuations in pollutant stability and toxicity, release of volatiles from solid fuels, densification of sludge and transformation of sludge into mainly inorganic compounds.
2.2 Anaerobic digestion
AD (Fig. 2) generates a biogas made up of 60–70% methane, 30–40% carbon dioxide and traces of other gases such as (H2S). The biogas has a calorific value of 28–39 MJ/Nm3 and can be upgraded by water scrubbing to produce a 97% methane biogas with a calorific content of up to 51 MJ/Nm3.
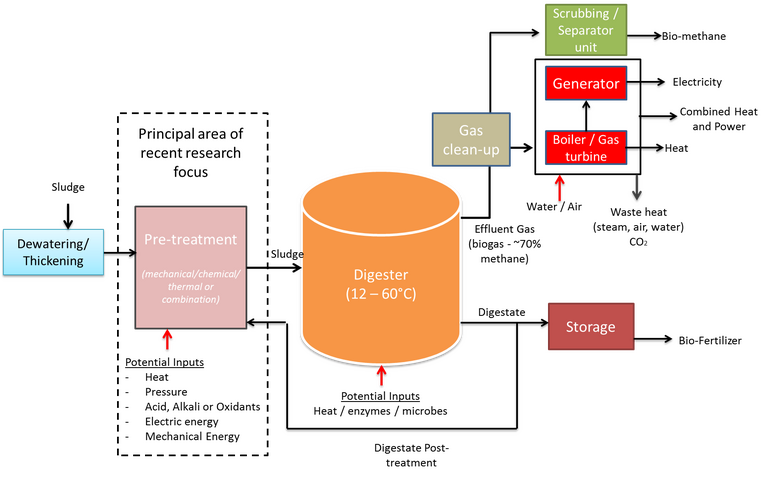
The latent energy in the biogas can potentially offset ~50% of the wastewater treatment plant energy consumption. Moreover, the supernatant product has a high nutrient content (phosphorus and nitrogen) which can be recovered for use as fertilisers for agricultural and soil reclamation purposes. However, the capital costs associated with the large plant size (relating to the long residence time) and the required degree of process control to maintain optimum biochemical conditions has limited implementation of AD to large treatment plants with the appropriate economy of scale.
Optimisation of the AD process to enhance the biogas yield has focused on effective process control, in particular temperature and pH, and pretreatment to enhance hydrolysis of the organic matter. Pretreatment methods have included chemical (oxidation or pH adjustment), mechanical (such as ultrasonication), and thermal (hydrothermal and microwave heating). These have all been demonstrated to enhance the biogas yield, and hydrothermal processes in particular have been successfully commercialised for this duty, but all pretreatment methods are subject to some constraints which ultimately impact on cost and technical efficacy (Table 2).
| Method | Advantages | Disadvantages |
|---|---|---|
| Acid or alkaline dosing | Simple to integrate | High operating cost (chemical consumption) |
| Low capital cost | Increased sludge mass | |
| Storage of hazardous materials | ||
| Ozonation | Flexible operation | High energy consumption |
| Reduced sludge volume | Possible mineralisation of cellular matter | |
| Ultrasonication | Low cost | High energy consumption |
| Simple maintenance | Scale-up challenges | |
| Microwave irradiation | Rapid heating | High energy consumption |
| Simple regulation | Scale-up challenges | |
| Hydrothermal treatment | Reduced sludge volume | High energy consumption |
| Improved sludge dewaterability | High capital cost | |
| Possible ammonia generation |
While incurring a large footprint and providing limited organic carbon conversion compared with thermochemical methods, AD is currently the most extensively used of the sludge resource recovery methods, with a number of different process configurations. The process benefits of energy neutral or positive operation overall due to the obviation of drying (Fig. 3) present significant advantages over the alternatives. Moreover, co-digestion with food waste has been shown to boost biomethane production and improve efficiencies generally. It has also been shown that pyrolytic post-treatment of the dried digestate can provide further energy efficiencies.
Anaerobic digestion − an introduction

2.3 Combustion
Combustion (or incineration), like AD, is an established method for sewage sludge stabilisation and resource recovery, recovering thermal energy while reducing the sludge volume by up to 90%. Unlike AD, a substantially dried feed is required (Fig. 4), with moisture content between 20 and 50%, and no useful combustible product is generated. Instead, as well as CO and CO2, the process produces harmful products such as oxides of nitrogen and sulphur (NOx and SOx) and suspended fly ash particles from furnace temperatures above 850°C. The flue gas, following thermal energy recovery, therefore requires extensive treatment prior to discharge.
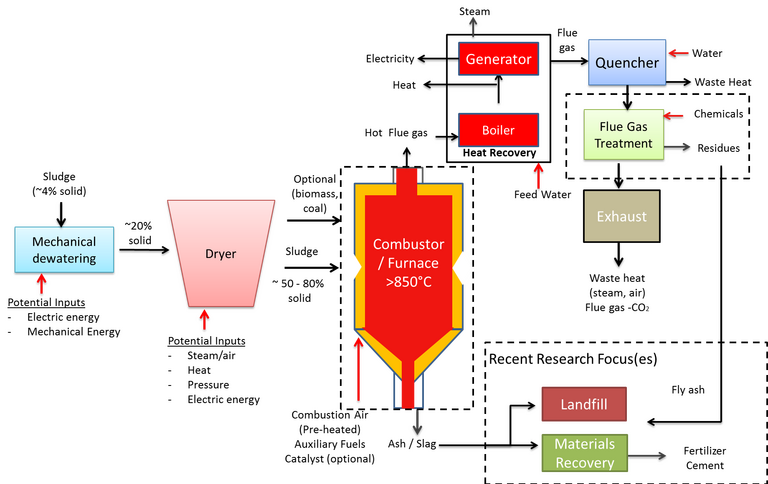
The reuse of the solid ash/fly ash product is dependent on its composition. Higher-value reuse applications such as soil amendment is only possible if the heavy metal content is sufficiently low. The ash can otherwise be used in construction as a lightweight aggregate, or else must be disposed of to landfill.
Developments in incineration have been focused on:
- improving combustion reactivity and completion by optimising reaction parameters, using catalysts and/or blending with other fuels such as coal oil shale and biomass
- reuse of the ash products as fertilizer or cement, and
- reduction of harmful emissions such as CO, NOx, SOx, polyaromatic hydrocarbons (PAH) and heavy metals.
As with AD, co-treatment with other materials has been explored as a means of increasing efficiencies. Specifically, co-combustion with the feed fuel in coal-fired power stations offers a means of both eliminating the high cost associated with dedicated sludge incinerator installations and reducing net carbon emissions from fossil fuel usage. In such cases, the technical and economic viability of combined- vs single-source combustion must be critically studied with reference to operational efficiency, and pollutant formation and emissions via the flue gas or ash products (Fig. 5).
newsletterSignup is not templated

2.4 Pyrolysis
Pyrolysis is the thermochemical treatment of sludge in the absence of oxygen at temperatures of 350–600°C and demands a feed having a moisture content below 10% (Fig. 6). The distribution of the pyrolysis product phases – the solid 'biochar', the liquid 'bio-oil' and gaseous products which include non-condensable combustible gases such as methane and hydrogen – can be controlled through adjusting the process operating temperature, heating rate and residence time. Normally, conditions are selected to promote bio-oil formation, since this provides a transportable fuel once refined.
The bio-oil is formed from condensable gaseous products from the pyrolytic process, which is mechanistically complex and involves a number of steps taking place simultaneously. The bio-oil calorific value is in the region of ~33 MJ/kg, significantly higher than the AD biogas CV. However, the bio-oil generated from sewage sludge can have a relatively high (>23%) moisture content and a large proportion (33%) of oxygen-containing organic compounds which both diminish the fuel quality and value.
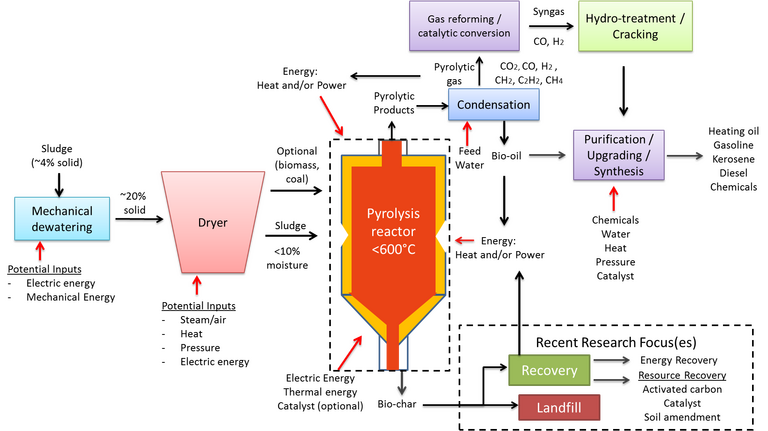
The bio-oil yield varies between 14 and 58%, depending largely on the feed sludge quality and the moisture content specifically. Yields appear to be maximised at temperatures of 450–550°C, implying that temperatures below this range are inadequate for optimal breakdown of the char phase while higher temperatures promote gas formation. Minimisation of the reaction residence time has thus been explored to suppress gas generation. This then limits the pyrolysis process to substantially dry sludge because of the negative influence of moisture on the reactor operating conditions, oil quality and increased amounts of non-condensable gases.
Bio-oil yields are also affected by the inorganic content of the sewage sludge. Metal oxides such as CaO and ZnO impede organic matter decomposition and so reduce the bio-oil yield, whilst some other transitional metal species can catalyse gas formation.
The lower temperatures and oxygen free conditions serve to limit the contamination of the bio-oil by toxic metals, other than trace amounts of the more volatile metals such as cadmium and mercury. These species instead arise in the biochar product, which represents at least 50% of the products generated and, like the bio-oil, has some calorific value. It can also potentially be used as an adsorbent, catalyst or as a fertiliser. However, since it retains the toxic metal content of the feed sludge, biochar requires further treatment for removal of these metals by leaching prior to possible reuse in much the same way as the ash product generated from incineration (Fig. 7).

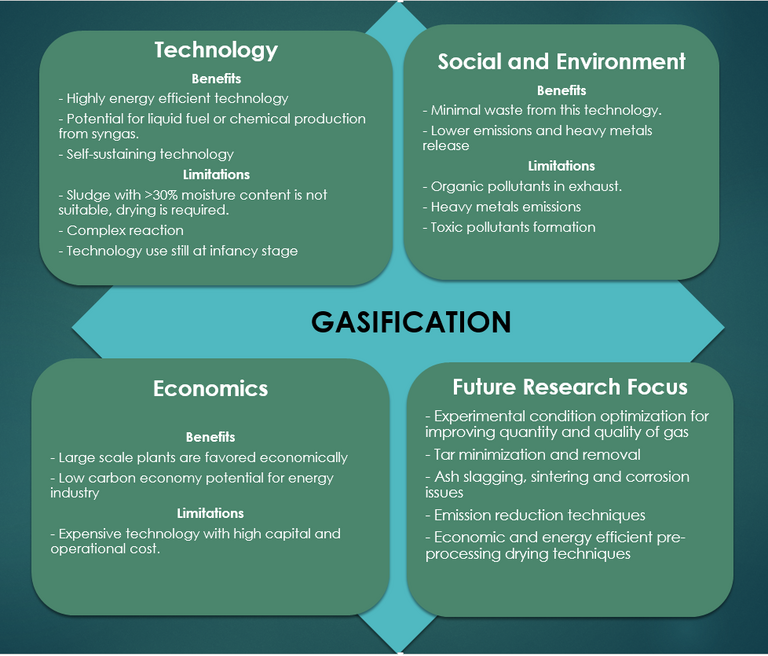
The complexities of the pyrolysis process, the requirement of a dry feed, and the negative impact of metals and other inorganic species all challenge the viability of the pyrolysis process. On the other hand, the reduced pollutant load in the gas stream, which contains no NOx or SOx species, and the generally favourable social, economic and environmental outlook as a potential zero waste technology has promoted considerable research activity in this area.
2.5 Gasification
Gasification is an extension of the pyrolysis process and, as such, the process flowsheet is almost identical (Fig. 8). The process operates at a higher temperature – 800–1000°C – in a partially oxygenated atmosphere. Under these conditions a 'syngas' product is formed, having a calorific value of 4–12 MJ/Nm3 depending on the feed sludge quality and process operating conditions.
The process is similar to combustion, other than the reduced moisture tolerance (<15 wt%) and the oxygen deficit. The reactor configuration is similar – normally based on a fluidised bed – and product gas can be combusted to generate heat for a CHP engine. The syngas, containing predominantly hydrogen, can otherwise be upgraded for further use. As such, a key objective in gasification design and operation is to maximise the syngas yield, in the same way as maximising the bio-oil yield represents the main aim in pyrolysis.
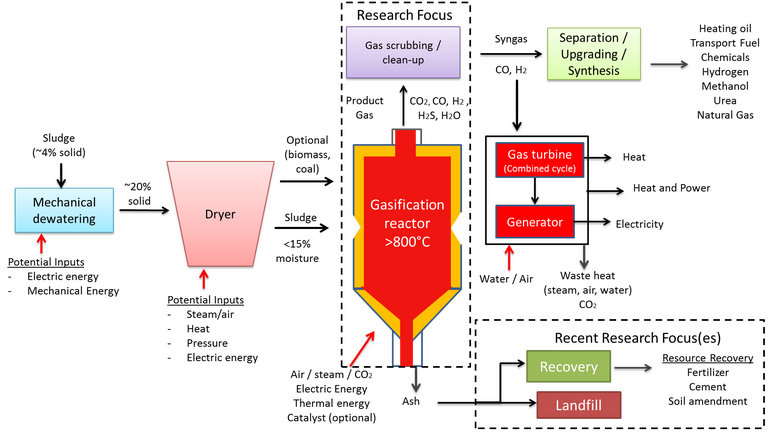
In addition to the parameters of importance in pyrolysis, namely residence time, temperature and catalytic constituents, a key operating parameter in gasification is the equivalence ratio (ER). The ER is the air-to-solids ratio, and impacts on the reactor temperature since the partial oxidation reaction is exothermic. This ratio is generally kept between 0.2 and 0.5 to maintain optimum conversion to syngas. Low ER values lead to incomplete gasification with high liquid ('tar') and solid ('char') fractions as end product, whereas higher values promote oxidation to CO2 and H2O at the expense of the syngas product.
Various catalysts (nickel, dolomite, zeolite, olivine and alumina) have been studied for reducing tar production and increasing H2 yields with some success. Some of these catalysts have also been shown to reduce the generation of pollutants such as NOx, though the deactivation of such catalysts at high temperatures from coke deposition remains a challenge.
The main challenges of sludge gasification are, as with pyrolysis:
- conversion to the required calorific product
- the negative impact of the inorganic constituents, and
- the pre-drying requirement.
The inorganic content of sewage sludge produces sintering, leading to agglomeration and clinker formation and increasing reactor shut down and maintenance. The high reactor temperatures and the low fusion temperature of the solid char product also produce operational problems associated with volatilisation and deposition of the sludge solids constituents. These include blockage of the product fuel flow, impeding both heat transfer and syngas quality, and contamination from deposited compounds containing toxic metals.
As with pyrolysis, a key issue is the fate of the toxic metals and their stability in the solid char product (Fig. 9). Whilst char is a potential resource, as with pyrolysis, the presence of tar is generally problematic since condensation of this phase leads to clogging, fouling and other downstream issues. Removal of tar, or suppression of its formation through catalysis or effective pre-drying of the sludge feed, adds to the process cost.
Finally, there is an additional byproduct issue with sludge gasification concerning sludges of high nitrogen and sulphur content. These components lead to ammonia, hydrogen cyanide and hydrogen sulphide formation, all of which are toxic and/or undesirable to varying degrees.
3. Conclusions
A comparison of the two established sludge technologies of anaerobic digestion (AD) and combustion with the two more novel oxygen-deficient thermochemical processes of pyrolysis and gasification for resource recovery from sewage sludge reveal major challenges to implementation of the novel technologies. This primarily relates to the constraints imposed by the sludge composition. In addition to the organic content, sewage sludge has a high moisture content and contains various inorganic constituents which influence:
- the process energy consumption, conversion efficiency and process maintenance requirements, and thus overall costs, and
- the purity of the final products, and thus the extent of downstream refining.
Against this, the conventional processes are also subject to a number of constraints. Anaerobic digestion, whilst requiring little or no pre-treatment of the sludge, demands extremely long processing times, provides limited conversion efficiencies, and generates a digestate stream which, whilst stabilised, requires further processing to render it environmentally benign. Improving the conversion to biogas requires pre-treatment which generally increases the overall process costs.
Combustion processes normally require drying, though not to the same extent as gasification and pyrolysis, and produce gaseous omissions which are environmentally onerous. Removal of the harmful pollutants from the flue gas stream adds significantly to the process cost. Moreover, the reuse options for the ash generated are currently limited to low-value applications.
Pyrolysis and gasification both provide rapid conversion of pre-dried sludge to a product having a significant calorific value. They are both subject to control issues which must be met to maximise energy conversion efficiency. This has motivated study of microwave heating, which appears to offer effective heating and some tolerance to sludge of moderately high moisture content, but such systems have scale-up issues and their energy conversion efficiency is low.
All of the technologies, however, appear to benefit from combined treatment with other biomass or otherwise carbon-rich materials. It also appears possible that application of the pyrolysis and gasification technologies downstream of the AD process to recover the residual resource in the digestate may also be economically viable.
Whatever the future may hold, it is crucial that the energy balance determinations take full account of all energy inputs – including that demanded for drying – as well as the outputs. Similarly, the environmental impact of all discharged streams, and in particular the fate of the toxic metals, must be encompassed in any broad-based comparative analysis of process options. It may well be that the trade-off between environmental impact and net energy demand will ultimately determine the preferred sludge-to-energy process option.






